Clinical Trial Assesses Safety and Tolerability of Escalating Doses of ANPD001 Autologous, Dopaminergic Neuronal Cell Replacement Therapy
SAN DIEGO, Jan. 13, 2025 /PRNewswire/ — Aspen Neuroscience, Inc. has successfully completed dose escalation and the first two cohorts of patients in the ASPIRO study, an ANPD001 Phase 1/2a clinical trial in Parkinson’s Disease (PD).
The Autologous-derived Study of a Parkinson’s Investigational Regenerative therapy in an Open-label trial (ASPIRO) was launched in 2024 to assess ANPD001 in patients with moderate to severe PD.
The study is examining escalating doses, with the primary endpoint being safety and tolerability of ANPD001. Secondary endpoints include improvement in “on” time, when patients experience periods of symptom control, and improvements in motor symptoms and quality of life based on standard PD rating scales.
“This is a major milestone for the first multi-patient, multi-center clinical trial of an autologous therapy for Parkinson’s disease,” said Edward Wirth III, MD, PhD, Chief Medical Officer of Aspen Neuroscience. “We are happy to announce that to date, ANPD001 and its delivery have been well tolerated, and no serious adverse events have been observed in the first two cohorts of patients in the ASPIRO study. All patients were discharged within 48 hours, per protocol. We are now advancing the program to investigate our new commercial formulation.”
ANPD001 is the most advanced autologous investigational new cell therapy in the United States for treating Parkinson’s disease. More information about the Phase 1/2a trial is available at clintrials.gov (NCT06344026).
The study trial includes patients 50–70 years of age, and excludes patients with cognitive impairment and other comorbidities that could preclude treatment. All enrolled patients are under the care of a movement disorder specialist.
About ANPD001
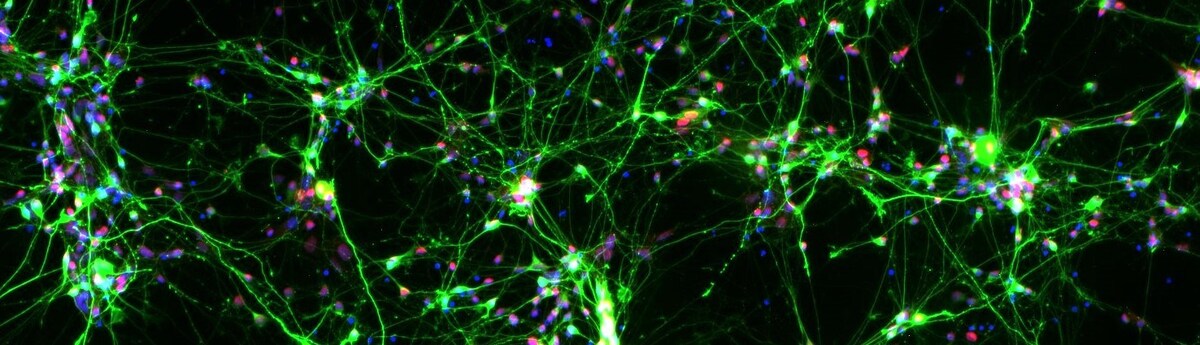
ANPD001 is an investigational autologous dopaminergic neuronal precursor cell (DANPC) therapy being studied as a regenerative treatment for PD. Aspen’s personalized approach means that patients do not require immunosuppressive drugs to counteract the body’s immune response against foreign cells.
Aspen’s manufacturing process starts from a small sample of the patient’s own skin cells, followed by reprogramming to induced pluripotent stem cells (iPSCs) and then differentiation of the iPSCs into DANPCs. These DANPCs are transplanted into the putamen, replacing cells that were lost or damaged due to disease. The quality of each person’s cells is assessed at every manufacturing stage using Aspen’s proprietary machine learning-based genomics tests.
ANPD001 has received Fast Track designation by the U.S. Food & Drug Administration (FDA).
About Aspen Neuroscience
Headquartered in San Diego, Aspen Neuroscience, Inc. is a clinical development-stage, private company focused on autologous regenerative medicine. The company’s patient-derived iPSC platform is used to create personalized therapies to address diseases with high unmet medical needs, beginning with autologous neuron replacement for PD.
Aspen combines cell biology with the latest machine learning and genomic approaches to investigate patient-specific, restorative cell treatments. The company has developed a best-in-class platform to create and optimize pluripotent-derived cell therapies, which includes in-house bioinformatics, manufacturing and quality control. For more information and important updates, please visit https://www.aspenneuroscience.com.
SOURCE Aspen Neuroscience, Inc.

Get the source article here