While Parkinson’s disease (PD) has a large and highly generalised therapeutic market, key opinion leaders (KOLs) interviewed by GlobalData have highlighted significant unmet needs within the field, including a lack of neuroprotective/disease-modifying therapies (DMTs) and therapies addressing non-motor symptoms.
Currently, there are no marketed DMTs for PD. All marketed PD products are symptomatic therapies, with levodopa therapies and dopamine agonists dominating the market due to their efficacy against core PD motor symptoms. The PD drug development pipeline across the seven major markets (7MM: France, Germany, Italy, Japan, Spain, the UK and the US) reveals a strong research focus on addressing these unmet needs through a diverse range of investigational mechanisms of action. The pipeline distribution highlights a strong emphasis on neuroprotective and disease-modifying agents, reflecting the field’s commitment to advancing treatments that could alter the course of the disease.
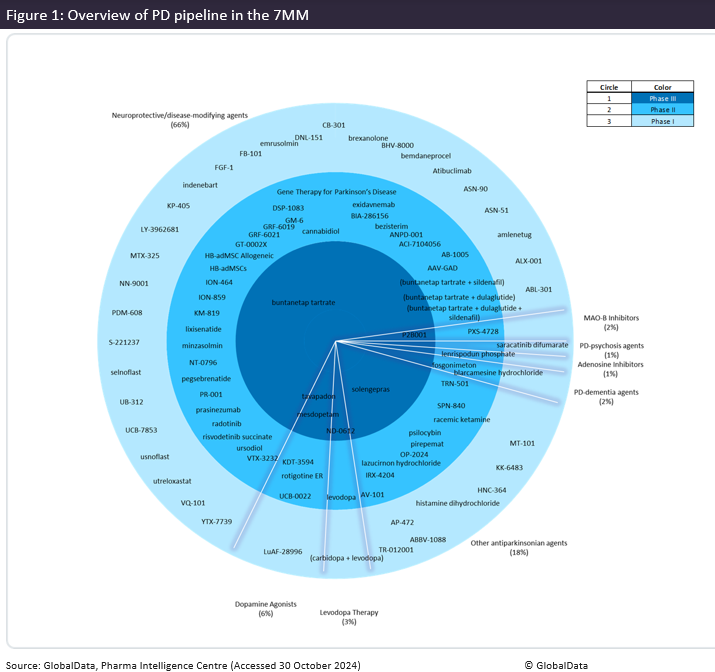
According to GlobalData’s drug database, there are 93 pipeline products in Phase I to Phase III development within the 7MM for the treatment of PD (Figure 1). Products that are being investigated for their neuroprotective or disease-modifying properties account for 66% of the pipeline. These investigational agents target key mechanisms implicated in the pathophysiology of PD, such as alpha-synuclein aggregation and neuroinflammation, to slow disease progression. Products in late-stage development include Annovis Bio’s Posiphen (buntanetap tartrate), which seeks to inhibit alpha-synuclein and is in Phase III development (NCT05357989) in the US and five major European markets (5EU: France, Germany, Italy, Spain and the UK), and BioVie’s Triolex (bezisterim), an inhibitor of inflammation mediators that has a Phase III trial planned in the US due to its neuroprotective potential.
Therapies targeting alpha-synuclein aggregation account for 26% of the pipeline DMTs. KOLs interviewed by GlobalData had divided opinions on the likely efficacy of anti-alpha-synuclein therapies. Some KOLs expressed strong skepticism, drawing attention to safety concerns due to the role of alpha-synuclein in healthy functioning, and the failure of Prothena/Roche’s prasinezumab to meet its primary endpoint in the Phase II PASADENA trial (NCT03100149). However, KOLs agreed on the need for research to focus on the development of DMTs, while highlighting that a potential challenge in their development is that the pathogenesis of PD can differ between patients.
KOLs also highlighted a particular unmet need and expressed enthusiasm for therapies addressing falls and non-motor PD symptoms. The PD pipeline demonstrates a significant research effort to address these needs within the 7MM. A total of 18% of the pipeline is classified as “other anti-Parkinsonian agents”, which emphasise symptom management beyond core motor symptoms. These other agents include Cerevance’s solengepras, currently under Phase III (NCT06553027) investigation in the US, which aims to improve postural instability in PD patients, IRLAB’s Pirepemat, which is under investigation at Phase IIb (NCT05258071) and Phase II for both postural instability and PD-dementia, and Silo Pharma’s psilocybin (Phase II), which targets cognitive and emotional impairment (particularly depression and anxiety) in patients with PD.
The focus on improving non-motor symptoms in PD patients is highlighted by the fact that 3% of the pipeline is specifically targeting PD-dementia and PD-psychosis. The distribution of the 93 pipeline agents in the 7MM shows a strong emphasis on neuroprotective therapies and DMTs, demonstrating an industry-wide shift toward therapies that could slow the progression of PD. Research into other anti-Parkinsonian agents, dopamine agonists and treatments for PD-related dementia highlights a comprehensive approach that aims to improve both motor and non-motor symptoms, addressing the broader spectrum of challenges faced by PD patients.
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Your download email will arrive shortly
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalData
Get the source article here