
A new study has identified polyphosphate as a likely “mystery density” within fibrils associated with University of Michigan has uncovered compelling evidence that may solve a fundamental mystery surrounding the structure of fibrils involved in Alzheimer’s, Parkinson’s, and other neurodegenerative diseases.
“We’ve seen that patients have these fibril structures in their brains for a long time now,” said Ursula Jakob, senior author of the new study. “But the question is what do these fibrils do? What is their role in disease? And, most importantly, can we do something to get rid of them if they are responsible for these devastating diseases?”
Although the new finding does not explicitly answer those questions, it may provide a missing piece of the puzzle for researchers that are trying to understand how these diseases work at a molecular level. And it’s clear that this more intimate understanding is needed, given the lack of Alzheimer’s treatment options, Jakob said.
The Food and Drug Administration has approved three new drugs for Alzheimer’s disease since 2021, but that was preceded by a 17-year stretch without any new approvals despite hundreds of clinical trials (even now, there are more than 100 drug candidates being evaluated).
“Given all these unsuccessful clinical trials, we must still be missing some important pieces of this puzzle,” said Jakob, a professor in the U-M Department of Molecular, Cellular, and Developmental Biology. “So the fundamental research that we and many others around the world are doing is critically necessary if we ever want to treat, much less eliminate, these terrible diseases.”
The mystery density
Researchers have long known fibrils—tiny tendrils assembled from invisibly small building blocks called amyloid proteins—are connected to a number of neurodegenerative diseases. But important questions have lingered about how these structures build up in the body and how they affect the progression of these disorders.
Our understanding of the fibrils continues to develop as scientists introduce new tools and methods to probe the structures more intimately. One of those innovations is known as cryogenic electron microscopy, or cryo-EM.
“This is a very sophisticated technique,” Jakob said. “With it, you can see what these fibrils look like in great detail.”
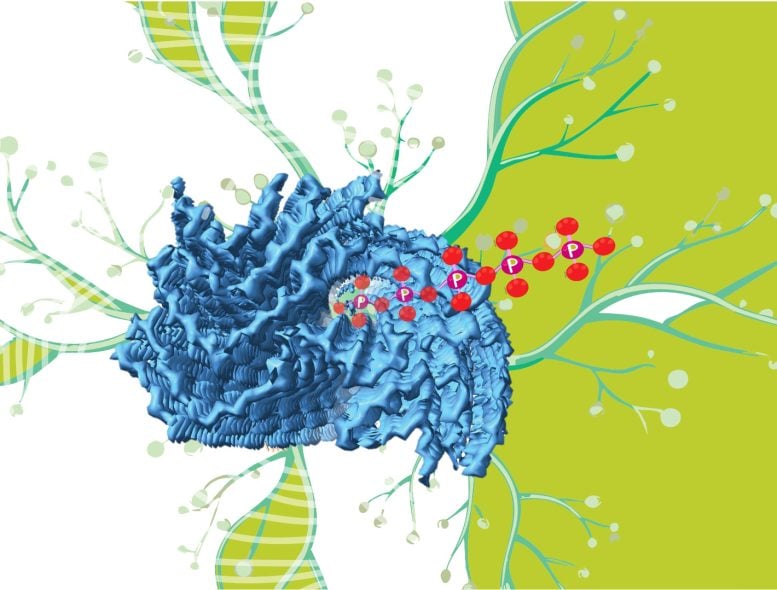
In 2020, an international team led by researchers in Cambridge using cryo-EM discovered a mysterious mass inside fibrils that were recovered from patients with a neurodegenerative disease called multiple system atrophy.
Although researchers could characterize the fibrils down to the individual amino DOI: 10.1371/journal.pbio.3002650
The work was supported by the Adblock test (Why?)



