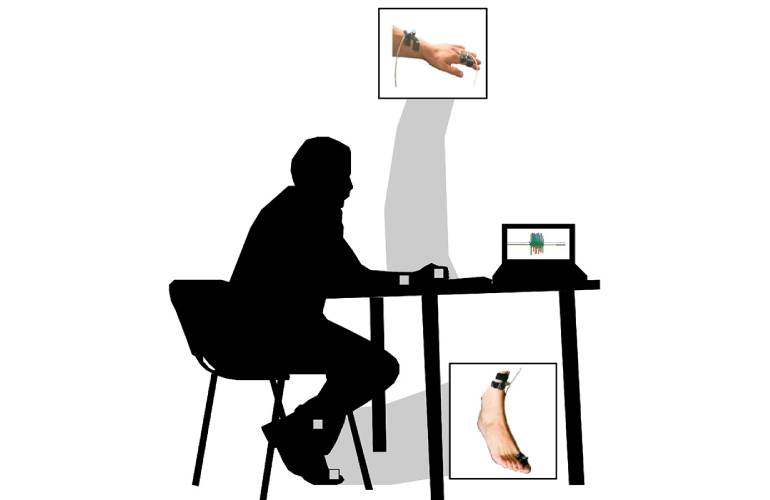
Researchers at the University of Illinois say low-cost, wearable sensors could increase access to care for Parkinson’s disease patients.
The team, along with clinical collaborators at Johns Hopkins, say new machine learning approaches and baseline data from healthy older adults improve the accuracy of the results from these sensors.
“This study demonstrates that the expansion of datasets with healthy older adult motion data and integration with deep learning approaches can help improve the accuracy of detecting differences in motor impairment in persons with Parkinson’s disease for use in future telemedicine sessions,” said study leader Manuel Enrique Hernandez, a professor of biomedical and translational sciences within the Carle Illinois College of Medicine at the University of Illinois.
Patients with Parkinson’s have to periodically undergo assessments to have their symptoms monitored. This time-consuming, in-person specialist visit often comes with limited availability, Hernandez said. Telemedicine evaluations could improve care but is hampered by a lack of quantifiable measurements.
The Illinois team set out to take on these issues by improving assessments with low-cost wearable sensors. They published results in the journal Sensors.
“Ideally, we’d have something that’s completely passive, gathering data as a person goes through their everyday environment and using that information to provide guidance on their overall motor function and progression of neurological symptoms,” Hernandez said. “But then you face the big challenge of how to parse all that information and make it useful to a clinician. That’s what led to our strategy of improving the machine learning, honing in on activities useful for assessment and looking at healthy individuals as a baseline.”
The researchers adapted the Movement Disorders Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale, a clinical assessment method. MDS-UPDRS outlines tasks that a patient would perform and qualitative observations a physician would make during an exam. It then organizes them into categories for scoring.
For the study, the investigators had patients perform tasks and muscle movements while wearing sensors to provide data for the categories scored by MDS-UPDRS. They used data from both patients with Parkinson’s disease and from healthy older adults to train the machine learning model. Hernandez said this helped build a comparative model to identify marked change in terms of motor function or motor impairment.
The researchers used a method of deep learning called pretraining to enhance the model. This helps to better identify important data points and filter out irrelevant ones. According to the team, pretraining improved the accuracy of identifying motor impairments in hand movement tasks by more than 12% over the current standard model. Additionally, data from healthy older adults improved the accuracy of assessing every task for the upper and lower body, excluding toe tapping.
Looking forward, the team seeks to collaborate with neurologists and clinicians to expand their model. They hope to find additional tasks that could quantitatively measure more information about the symptoms of Parkinson’s, like tremor.
“There’s a very large need for us to better understand and better quantify changes that are ongoing with Parkinson’s disease,” Hernandez said. “The ability to use wearable sensors while performing activities that are part of the course of clinical evaluations in a telehealth setting might open up the doors for more objective, more quantifiable information that can help direct treatment for individuals with Parkinson’s disease and hopefully improve quality of life moving forward.”