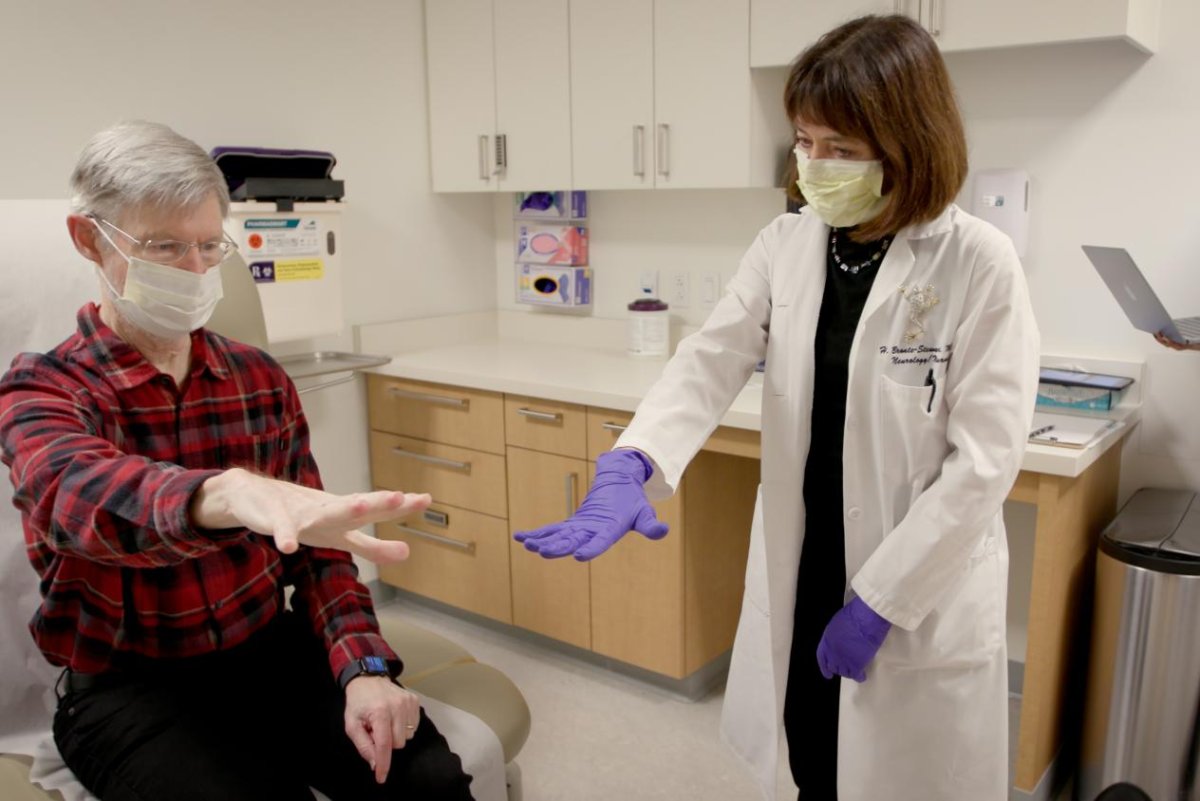
ST. PAUL, Minn., March 21 (UPI) — Parkinson‘s disease patients and advocates are marking the start of a new era for treatment of the illness, as a doctors deploy a breakthrough neurological device for the first time in the United States.
A team at Stanford University School of Medicine in Palo Alto, Calif., was set to connect two Parkinson’s patients to a new type of deep brain stimulation device, or “brain pacemaker,” developed by Medtronic Inc., on Friday in a ceremony to hail what they say is the beginning of a new paradigm in long-standing efforts to control sufferers’ debilitating tremors.
The device, called the BrainSense Adaptive deep brain stimulator, was approved by the Food and Drug Administration on Feb. 24 after a decade of development, testing and trials, and is now poised to make its clinical debut.
This brings hopes that, for the first time, a “DBS” device will be able to provide personalized brain neuromodulation in a way that “adapts” to patients’ changing needs on an around-the-clock, real-time basis.
The procedure will be led by Dr. Helen Bronte-Stewart, the John E. Cahill Family Professor at Stanford Medicine’s department of Neurology and Neurological Sciences, who also helmed the clinic trials for the device.
She told UPI the occasion will honor the years of “hard work” that went into the development of a device that has the potential to change the everyday reality of Parkinson’s sufferers.
“The exciting thing is to know that whenever somebody used this device as part of our research trials, they knew it was just for research, that it may not be part of their actual clinical care,” Bronte-Stewart said. “Now, once they’re on the adaptive DBS, they’ll know ‘this is it,’ it’s part of their clinical care and they can stay on it forever.”
The BrainSense device, in essence, is a “smarter version” of the decades-old deep brain stimulation technology that uses electrical pulses to regulate abnormal brain activity in people with Parkinson’s disease. DBS technology, in combination with medication, has helped ease tremors, stiffness and slowed movements for years.
However, traditional DBS tech has a downside. It is essentially a “blunt instrument,” or a one-size-fits-all solution, pumping out the same level of pulses to electrodes implanted in the brain no matter how a patient’s system changes over the course of time, much as cardiac pacemakers once did for the heart. That meant DBS carries too many side effects or is not effective for some patients.
But now, also like pacemakers, technological advances allow the devices to individualize their functionality. In the case of Medtronic’s new product, it can use a person’s individual brain signals to control the electric pulses it delivers in real time, making it much more precise and efficient than older DBS methods.
The biggest challenge to overcome was the development of the precise and accurate algorithms needed to make the device work in a safe manner.
“A lot of work was put into which inputs to use, which is partly why it took 10 years to develop,” Bronte-Stewart said. “You don’t want to feed the wrong signal into these automated algorithms because the system is going to adjust itself based on rhythm it’s sensing.
“That was the big learning curve, just as with the development of smart cardiac pacemakers. What are signals that the algorithms will act off of to go in the right direction?”
Another advantage of the new technology is that it will allow for a better balance between the use stimulation and medication to treat Parkinson’s tremors.
“With continuous DBS, it couldn’t adjust accordingly when medication kicks in,” she said. “You then have overtreatment, and when the medication wears off, you then have undertreatment.
“So, the first way this is going to help is that we’ll have a system that measures that brain arrhythmia, and if it senses the medication is doing its work, then the DBS will adjust its amplitude down.”
In announcing the FDA approval last month, Medtronic called it the largest-ever commercial launch of brain-computer interface technology by several magnitudes.
It came after an international study representing the largest and longest assessment of adaptive DBS, an effort led by Stanford, the University of California-San Francisco, Massachusetts General Hospital and Amsterdam University Medical Center.
Friday’s scheduled initial clinical rollout of the device brought praise from the American Parkinson Disease Association, which called it a major moment in the battle against the disease.
“It is incredibly exciting for the [Parkinson’s disease] community that this technology is now available clinically, as it has the potential to optimize symptom management to a degree that has not been possible before with standard DBS,” APDA chief mission officer Dr. Rebecca Gilbert said in a statement to UPI.
“Parkinson’s disease and its symptoms can vary quite a bit from person to person, so the more personalized and customized treatment can be to each individual, the better.”
Gilbert noted existing DBS systems can “make a life-changing difference in my patients who suffer from significant tremors or motor fluctuations.” Therefore, she added, “it stands to reason that adaptive DBS would result in better symptom management for each individual, and I am eager to see how that unfolds.
“This has the potential to be a game-changer.”



