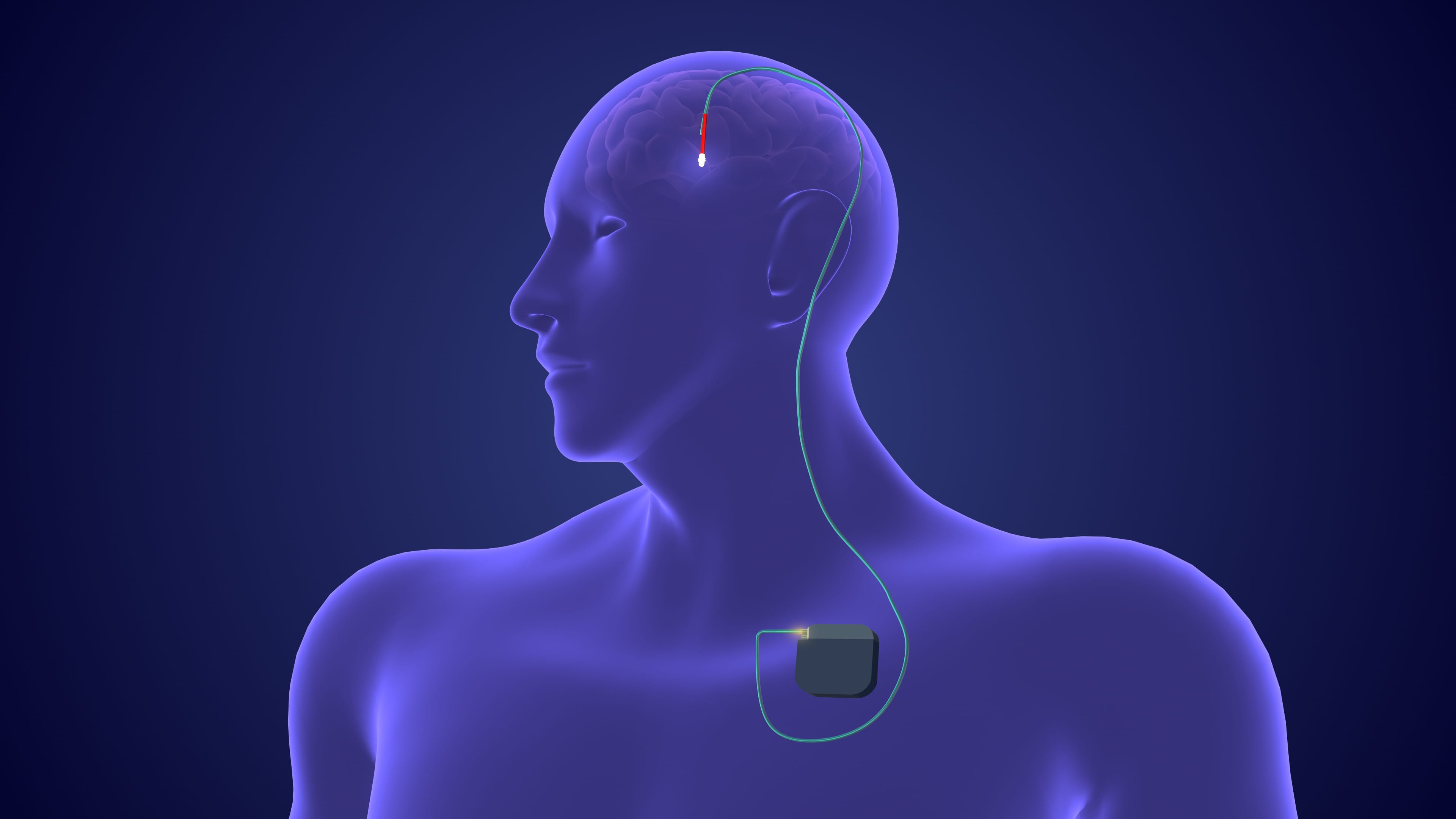
Neuromodulation has undergone significant advancements over recent decades in the treatment of Parkinson disease (PD). Among the growing array of invasive and non-invasive approaches, deep brain stimulation (DBS) remains the most well-established intervention.1-3
“DBS represents the cornerstone of neuromodulation therapy in PD, with more than 150,000 patients treated worldwide to date,” said Vibhor Krishna, MD, associate professor in the department of neurosurgery at the University of North Carolina School of Medicine.4
The US Food and Drug Administration (FDA) has approved DBS for stimulation in the subthalamic nucleus, globus pallidus pars interna, and ventral intermediate nucleus of the thalamus, with clinical outcomes varying by target site.5
Symptom Improvement and Proposed Mechanisms
Clinical outcomes with DBS have been consistently favorable across PD symptoms. “Modern DBS treatment typically achieves a 50% or greater reduction in symptoms and provides patients approximately 4 additional hours of ON time each day,” Dr Krishna said.6,7 “Importantly, DBS also offers flexibility in medication management personalized to each patient’s clinical needs, enabling either a significant reduction in dose if the medication burden is high, or a safe escalation of doses in patients previously limited by levodopa-induced dyskinesias.”
Symptoms most responsive to DBS include bradykinesia, rigidity, and tremor. The effects are “similar to the benefits of a good dose of levodopa, with additional tremor control,” said A. Enrique Martinez-Nunez, MD, movement disorders fellow in the department of neurology at the University of Florida College of Medicine. Dyskinesias associated with levodopa are also reduced, either through decreased medication requirements with subthalamic nucleus stimulation or through direct suppression via stimulation of the globus pallidus pars interna. However, other symptoms have shown variable improvements with DBS. 7 “Balance and gait typically do not improve unless tied to rigidity or bradykinesia, and freezing of gait can improve with specific settings in a small subset of patients,” Martinez-Nunez noted, adding that cognitive symptoms may worsen with DBS.
The precise mechanisms of DBS in PD have yet to be fully defined. Early hypotheses proposed that the therapy created a reversible lesion at the electrode implantation site, thus reducing activity in that structure. Subsequent work, however, pointed to more complex mechanisms, according to Nicole C. Swann, PhD, assistant professor in the department of human physiology at the University of Oregon. “An alternative mechanism is that PD is characterized by excessive synchronized activity in the beta range (13-30 Hz) throughout basal ganglia-thalamo-cortical networks, and this synchronization may constrain neural activity into inflexible patterns,” she explained.8 “The stimulation delivered by DBS can then break up this synchronization, which allows for resumption of dynamic neural activity.”
Stimulation at the subthalamic nucleus or globus pallidus pars interna has been shown to suppress the abnormally increased activity in the beta band, and “treatment with levodopa achieves the same effect, so we know we are targeting the same pathological circuit,” Dr Martinez-Nunez noted.7 “More specifically, the electrical field can entrain axons to fire at a specific rate, create antidromic potentials to raise the stimulation threshold of the neuron’s body, and stimulate axons that are nearby, and it also leads to changes in blood vessel permeability and local blood flow.”
Indications and Risks of Utilizing DBS in PD
Careful patient selection and thorough risk assessment are critical components in determining the DBS suitability in PD. Dr Krishna emphasized the importance of a comprehensive, multidisciplinary evaluation, stating that “ideal candidates include PD patients experiencing motor fluctuations on levodopa therapy, medication-refractory tremors, or quality-of-life-limiting dyskinesias.” He added, “Prerequisites for DBS include good general health with well-managed comorbidities and the absence of significant cognitive or psychiatric conditions that could compromise outcomes.” In addition, Dr Swann noted that patients should be able to return for the frequent stimulation adjustments and long-term monitoring that DBS treatment requires.
Dr Martinez-Nunez identified 2 main risk categories patients should understand before DBS treatment: surgical risks and stimulation side effects. Among the most serious surgical complications are cerebral hemorrhage and hardware infection. “These are rare, but they are seen in every center that does enough of these cases,” he noted. Reported hemorrhage rates range from 0.6% to 3.3% per procedure, and there is a 2.8% infection rate following DBS device placement.9.10
While stimulation-induced side effects are often transient and may be avoided by using certain programming strategies, Dr Martinez-Nunez cautioned that “some side effects from stimulation can be unavoidable.” Gait and cognitive impairments are the most common long-term stimulation-related side effects,11 underscoring the importance of careful patient selection. “Patients who have significant cognitive impairment or gait impairment that does not respond to levodopa should proceed carefully given these risks.”
Barriers to DBS Access
While DBS has become the most widely used surgical treatment in patients with PD,12 ongoing barriers limit wider access.
“Notable disparities exist in treatment access, particularly affecting rural populations, women, and minority groups,” Dr Krishna stated. Women, racial and ethnic minorities, and patients with lower socioeconomic status are less likely to undergo DBS compared with other groups. Additionally, more than one-third of PD patients with DBS have reported difficulty accessing clinics, often due to factors such as travel distance.13,14
“DBS is not available at all hospitals, and having long-term access to a clinician to provide programming adjustments as needed for years after surgery can be a barrier – especially for patients who live in rural areas,” Dr Swann said.14
Ongoing Developments and Future Directions
Beyond current clinical use, DBS technology continues to develop, with improvements being made both in the devices themselves and in stimulation delivery. Dr Swann noted enhancements such as “rechargeable batteries, segmented electrode leads that allow current steering, and more precise targeting and customization of stimulation.”
Adaptive stimulation, which enables adjustments based on feedback signals, is another major advancement.15 “Candidate feedback signals include brain signals recorded from the DBS electrode itself or from a separate electrode, or signals from other sensors such as accelerometry,” Dr Swann noted. A 2024 pilot trial demonstrated that adaptive DBS improved motor symptoms and quality of life compared with conventional stimulation in 4 patients with PD. Multiple clinical trials investigating adaptive DBS are currently underway.16
Additionally, “Technique optimization increasingly incorporates advanced neuroimaging, including tractography, for more precise stimulation targeting,” Dr Krishna said.17
“We are also discovering how stimulating in different ways can control different symptoms that we thought impossible to treat in the past,” added Dr Martinez-Nunez. “For example, some patients with freezing of gait can improve if we stimulate past the subthalamic nucleus, in the substantia nigra pars reticulata, at a lower stimulation frequency than we normally do, and we have found that stimulating in the most ventral part of the [globus pallidus pars interna] improves dystonia.”
Along with continuing efforts to further improve motor symptoms in PD via neuromodulation, researchers are focusing on the non-motor symptoms that many patients with advanced PD consider more burdensome than their motor symptoms, according to Dr Martinez-Nunez.18 “We are looking for treatments that improve other disabling symptoms, like apathy, cognitive impairment, anxiety, and impulse control disorders.”
Finally, researchers are investigating various forms of non-invasive neuromodulation, including transcranial magnetic stimulation, transcranial direct current stimulation, transcranial alternating current stimulation, and transcranial interference stimulation. These techniques may ultimately “serve as a trial option before considering surgical DBS,” Dr Martinez-Nunez said.
References:
- Krishna V, Fasano A. Neuromodulation: update on current practice and future developments. Neurotherapeutics. 2024;21(3):e00371. doi:10.1016/j.neurot.2024.e00371
- Krauss JK, Lipsman N, Aziz T, et al. Technology of deep brain stimulation: current status and future directions. Nat Rev Neurol. 2021;17(2):75-87. doi:10.1038/s41582-020-00426-z
- Cole RC, Okine DN, Yeager BE, Narayanan NS. Neuromodulation of cognition in Parkinson’s disease. Prog Brain Res. 2022;269(1):435-455. doi:10.1016/bs.pbr.2022.01.016
- Sankary LR, Ford PJ, Machado AG, Hoeksema LJ, Samala RV, Harris DJ. Deep brain stimulation at end of life: clinical and ethical considerations. J Palliat Med. 2020;23(4):582-585. doi:10.1089/jpm.2019.0129
- National Institute of Neurological Disorders and Stroke. Deep brain stimulation (DBS) for the treatment of Parkinson’s disease and other movement disorders. Updated December 3, 2024. Accessed January 27, 2025.
- França C, Carra RB, Diniz JM, Munhoz RP, Cury RG. Deep brain stimulation in Parkinson’s disease: state of the art and future perspectives. Arq Neuropsiquiatr. 2022;80(5 Suppl 1):105-115. doi:10.1590/0004-282X-ANP-2022-S133
- Martinez-Nunez AE, Justich MB, Okun MS, Fasano A. Emerging therapies for neuromodulation in Parkinson’s disease. Neurotherapeutics. 2024;21(3):e00310. doi:10.1016/j.neurot.2023.e00310.
- Weerasinghe G, Duchet B, Bick C, Bogacz R. Optimal closed-loop deep brain stimulation using multiple independently controlled contacts. PLoS Comput Biol. 2021;17(8):e1009281. doi:10.1371/journal.pcbi.1009281
- Walker RB, Grossen AA, O’Neal CM, Conner AK. Delayed hemorrhage following deep brain stimulation device placement in a patient with Parkinson’s disease and lupus anticoagulant syndrome: illustrative case. J Neurosurg Case Lessons. 2022;4(3):CASE2262. doi:10.3171/CASE2262
- Jung IH, Chang KW, Park SH, Chang WS, Jung HH, Chang JW. Complications after deep brain stimulation: A 21-year experience in 426 patients. Front Aging Neurosci. 2022;14:819730. doi:10.3389/fnagi.2022.819730
- Buhmann C, Huckhagel T, Engel K, et al. Adverse events in deep brain stimulation: a retrospective long-term analysis of neurological, psychiatric and other occurrences. PLoS One. 2017;12(7):e0178984. doi:10.1371/journal.pone.0178984
- Lee DJ, Dallapiazza RF, De Vloo P, Lozano AM. Current surgical treatments for Parkinson’s disease and potential therapeutic targets. Neural Regen Res. 2018;13(8):1342-1345. doi:10.4103/1673-5374.235220
- Bishay AE, Hughes NC, Zargari M, et al. Disparities in access to deep brain stimulation for Parkinson’s disease and proposed interventions: a literature review. Stereotact Funct Neurosurg. 2024;102(3):179-194. doi:10.1159/000538748
- Esper CD, Merola A, Himes L, et al. Necessity and feasibility of remote tele-programming of deep brain stimulation systems in Parkinson’s disease. Parkinsonism Relat Disord. 2022;96:38-42. doi:10.1016/j.parkreldis.2022.01.017
- Swann NC, de Hemptinne C, Thompson MC, et al. Adaptive deep brain stimulation for Parkinson’s disease using motor cortex sensing. J Neural Eng. 2018;15(4):046006. doi:10.1088/1741-2552/aabc9b
- Oehrn CR, Cernera S, Hammer LH, et al. Chronic adaptive deep brain stimulation versus conventional stimulation in Parkinson’s disease: a blinded randomized feasibility trial. Nat Med. 2024;30(11):3345-3356. doi:10.1038/s41591-024-03196-z
- Kamagata K, Andica C, Uchida W, et al. Advancements in diffusion MRI tractography for neurosurgery. Invest Radiol. 2024;59(1):13-25. doi:10.1097/RLI.0000000000001015
- van der Meer F, Jorgensen J, Hiligsmann M. Burden of non-motor symptoms of Parkinson’s disease: cost-of-illness and quality-of-life estimates through a scoping review. Expert Rev Pharmacoecon Outcomes Res. 2025;25(1):17-27. doi:10.1080/14737167.2024.2390042
Get the source article here


