One of the big questions about Parkinson’s disease is when and how it first takes hold?
We have long operated on the belief Parkinson’s begins when certain brain cells begin to die. And keep dying.
These cells – dopaminergic neurons – are the main source of the neurotransmitter dopamine, which plays a role in pleasure, motivation and learning.
Their degeneration is widely accepted as the first event that leads to Parkinson’s.
The big hope has been to somehow intervene, probably pharmaceutically, to catch Parkinson’s early. That is, before these cells start to die.
This would mean identifying what happens in the brain beforehand.
US researchers may have done it
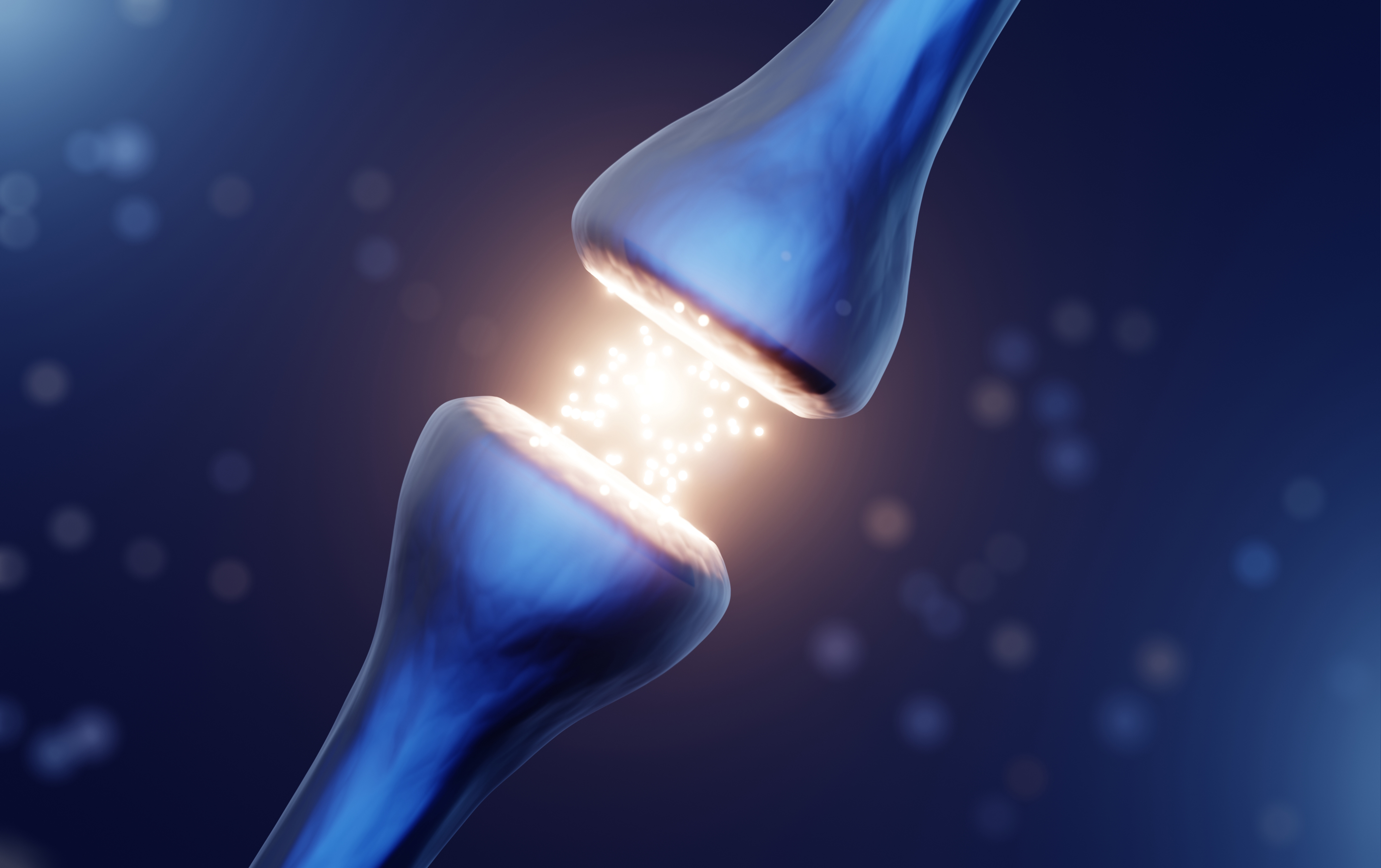
A study from Northwestern University Feinberg School of Medicine has found that “a dysfunction in the neuron’s synapses” is where the real trouble starts.
Synapses are the tiny connections between the neurons in your brain. They enable messages to be passed along.
The researchers suggest that this dysfunction in the synapses is what happens before the cells (neurons) begin to die.
They say the misfunctioning synapses is what causes the damage to loss of dopamine-making cells. This in turn leads to cell death.
Further, these early processes can begin decades before the disease presents itself.
Target for treatment
The scientists suggest that targeting these dysfunctional synapses, the tiny gaps, might be more effective pathway for treatment.
“We showed that dopaminergic synapses become dysfunctional before neuronal death occurs,” said lead author Dr Dimitri Krainc, chair of neurology at the school and director of the Simpson Querrey Centre for Neurogenetics.
You Might Like
“Based on these findings, we hypothesise that targeting dysfunctional synapses before the neurons are degenerated may represent a better therapeutic strategy.”
What’s causing the disruption?
It appears that faulty interaction of two genes – Parkin and PINK1 – is what causes the dysfunction of the synapses.
According to a statement from Northwestern, these genes “work to recycle mitochondria, the energy producers of the cell, that are too old or overworked”.
When things are working properly, PINK1 activates Parkin to move the old mitochondria into the path to be recycled or disposed of.
People who carry mutations in both copies of either PINK1 or Parkin develop Parkinson’s disease because of ineffective recycling of mitochondria.
The story of two sisters
Two sisters with the disease were of help in this research.
They were born without the PINK1 gene, “because their parents were each missing a copy of the critical gene”.
This put the sisters at high risk for Parkinson’s disease: but “one sister was diagnosed at age 16, while the other was not diagnosed until she was 48”.
The sister who was diagnosed at 16 “also had partial loss of Parkin, which, by itself, should not cause Parkinson’s”.
A big question
“There must be a complete loss of Parkin to cause Parkinson’s disease,” Krainc said.
“Why did the sister with only a partial loss of Parkin get the disease more than 30 years earlier?”
This question led to a new understanding of the Parkin gene: It has another important job that was previously unknown. It controls dopamine release. That’s a big finding in Parkinson’s research.
Northwestern scientists see an opportunity: Boost Parkin and the potential to prevent the degeneration of dopamine neurons.
“We discovered a new mechanism to activate Parkin in patient neurons,” Krainc said.
“Now, we need to develop drugs that stimulate this pathway, correct synaptic dysfunction and hopefully prevent neuronal degeneration in Parkinson’s.”
Hopefully.


